
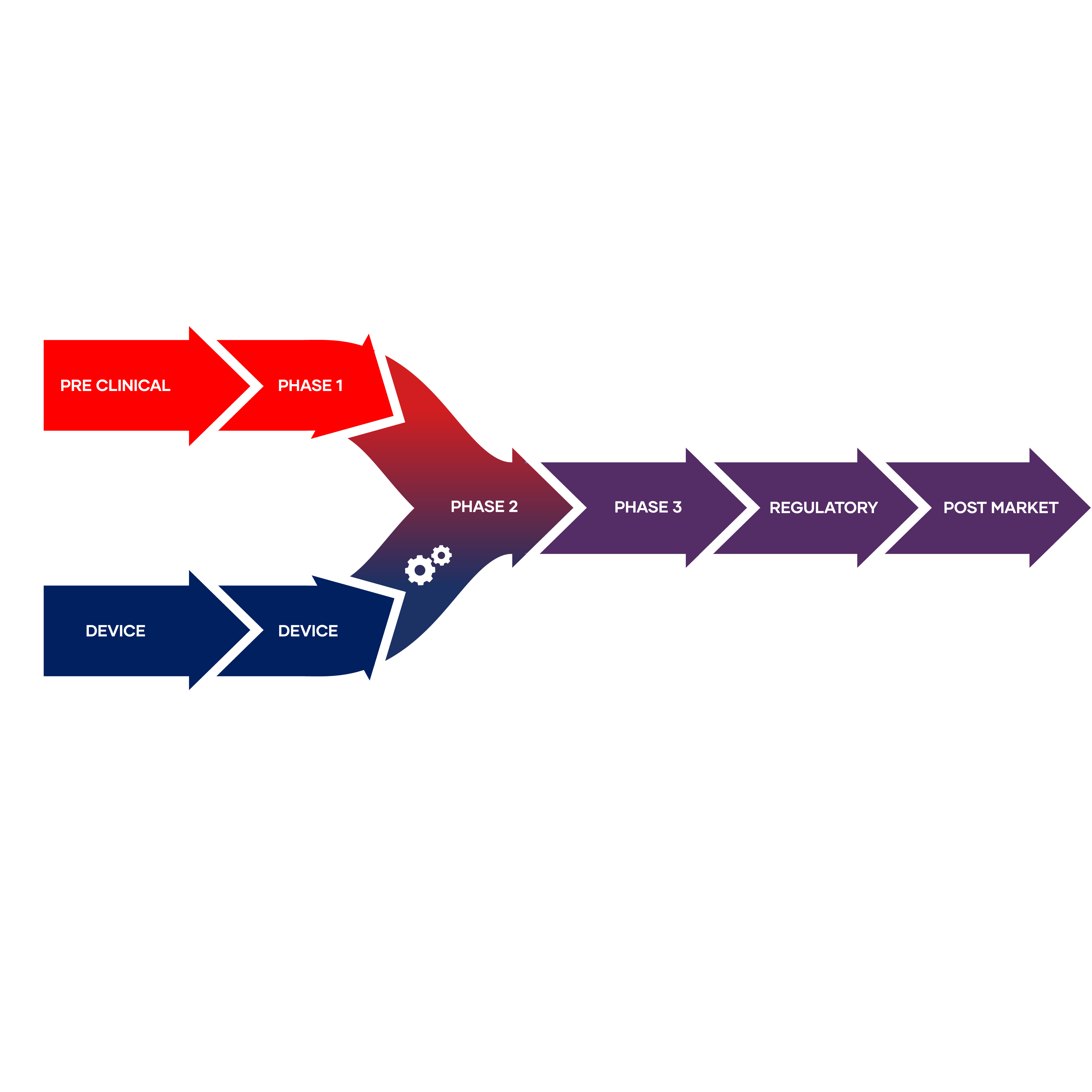
Planning for Device Success During Pre-Clinical / Phase I
Initiating device planning activities during Phase I is crucial to ensuring your combination product is ready for the next phase of clinical trials. EdgeOne Medical offers guidance to help emerging pharma companies confidently prepare for phase II by leading device selection processes, defining development strategies, and anticipating discussions needed with regulatory agencies.
Time to Start Planning
Many important decisions are made early in the program that can have a lasting impact on the development timeline and regulatory burden of the Combination Product. That's why, it's never too early to start looking ahead. EdgeOne Medical offers services necessary to help emerging pharma companies establish their device strategies and prepare for phase II device related activities.
Integrating the Device into the Drug Development Program
Most emerging pharma companies recognize they need a device to support the delivery of their product. But, once they have a device in mind, they start spinning their wheels uncertain of where to go from there and what questions to ask.
With EdgeOne’s expert guidance we’ll make sure to set you up for success by getting grounded in your current state and defining the device integration pathway into your program.
.jpg?width=1080&height=1080&name=file%20(1).jpg)
Pre-Clinical / Phase I Device Development Services
The critical device related activities performed during this stage are focused on device selection, planning for integration of device constituent and determining whether there are device related questions that need to be aligned with regulatory agency. In support of these activities, the most common services performed by EdgeOne Medical during pre-clinical / phase I clinical trial are provided below.
Device Selection & Development Strategies
We help you choose the appropriate device configuration for your drug product and identify development and manufacturing strategies that align with your technical and business goals.
Quality Management System Gap Assessment
We’ll review your existing Quality Management System against the required device development SOP’s, identify the gaps and define a phase appropriate plan to resolve gaps.
Early Drug-Device Feasibility Testing
Prior to finalizing device selection, we conduct preliminary test evaluations of device with drug, allowing you to identify potential issues early.
Device Strategy for Regulatory Interactions
Unsure what device questions to raise in early regulatory agency interactions? We’ll help you develop a strategy to ensure you address the right topics while demonstrating awareness of regulations and device processes.





The EdgeOne Advantage
At EdgeOne Medical, we help you de-risk your entire development program. By planning early, aligning device strategy with your drug, and engaging with the regulatory agencies at the right times, we ensure your transition to Phase II is as smooth as possible.
Common Questions in Phase I
We understand that addressing the device constituent can be confusing, especially when your internal team has limited device experience. Here are some of the most frequently asked questions from emerging pharma companies and how EdgeOne Medical can help..
-
The earlier, the better! During Phase I is the ideal time to start the process of evaluating and selecting the right device configuration for your combination product. While the device isn’t needed until phase II trials, the component and fill lead times are key factors driving availability of test data needed for a submission. Minimize delays to your phase II clinical trials by starting device conversations as early as you can. Contact us so we can help guide you through the process.
-
No, it is never too soon to start testing. We always recommend conducting feasibility evaluations as early as possible in the process to help de-risk the development program. A little data goes a long way! Just keep in mind that the feasibility testing data cannot be included in your submission package. The formal testing program to support your submission package needs to be aligned with appropriate device development activities. Need more details? Our team is just a message away.
-
Yes, some level of documentation is required. How much? The answer to this question is … it depends! The question that governs the magnitude of device development activities beyond the basic compatibility of the device with the gene is dependent upon regulatory assessment of your intended use compared to the cleared device intended use. Not sure how to perform this regulatory assessment? Reach out to see how we can assist you.