
Sailing Through Phase II/III Device Development
At this critical stage, device development must comply with device regulations while aligning with your drug development timeline. EdgeOne Medical’s team of experts is here to act as your device team and ensure your device program runs smoothly and efficiently.
Where the Heavy Lifting Happens
Preparing for phase II/III trials is when the device development activities really take off. At this stage in the program, compliance to regulations is expected and coordination and completion of activities to submission timeline is paramount.
Learning the Language of Devices
The device development process utilizes its own terminology when describing the process and activities. Design Inputs, Design Outputs, and Design Controls are an example of a few key device terms. For drug developers, this new language can create confusion.
EdgeOne Medical already speaks the language of device developers and is ready to help interpret and translate on behalf of your drug development program.
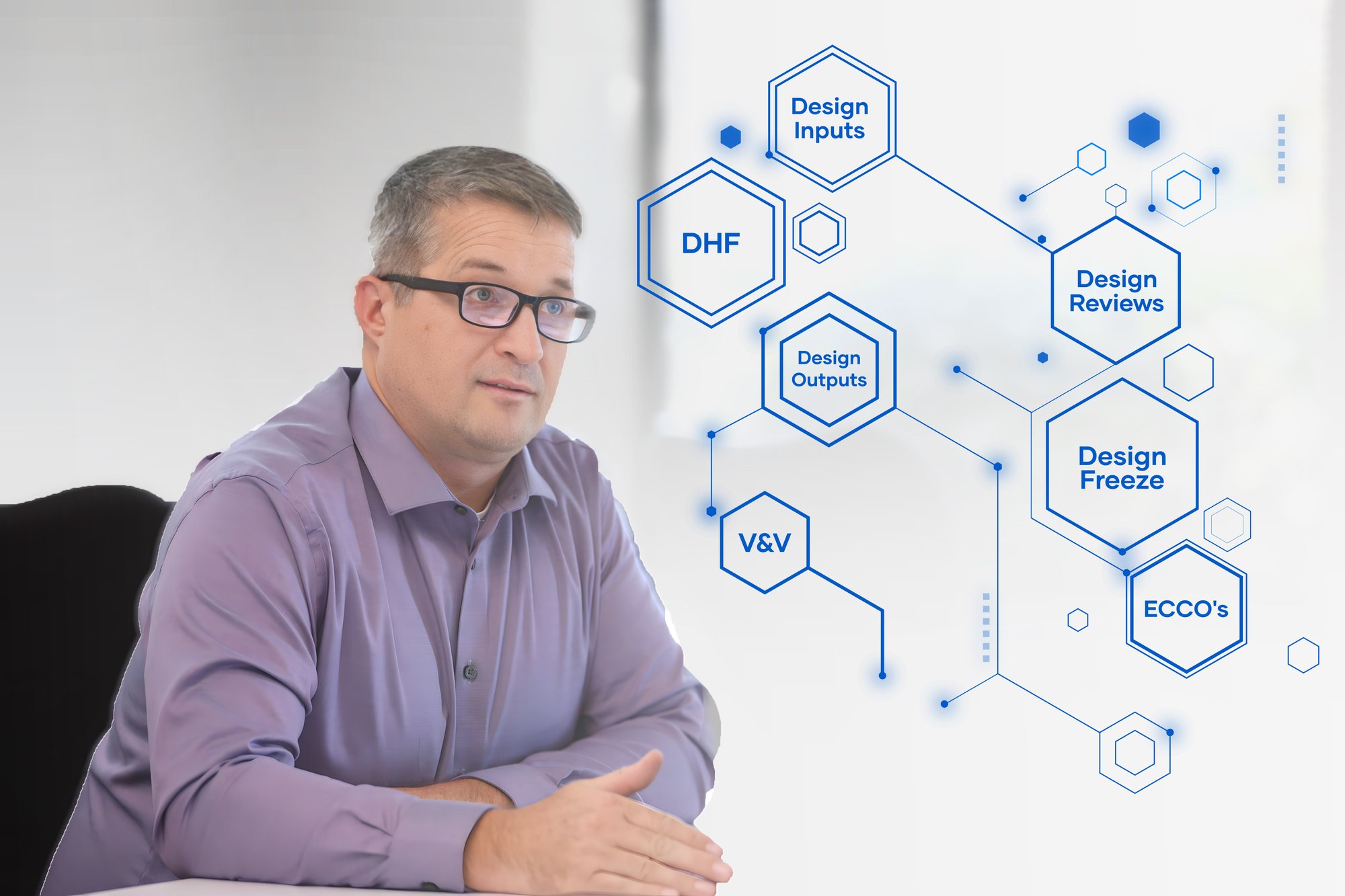
Phase II & III Device Development
Preparing for Phase II and III clinical trials comes with lots of moving parts, and we understand how challenging it can be to keep everything in sync. At EdgeOne Medical, we tailor our services to the unique needs of emerging pharma, guiding you through the regulations, building your QMS, and preparing for Phase II and III clinical trials—so you can focus on drug development activities.
Design Control Compliance
Navigating FDA 21 CFR Part 4 and ISO 13485 can be tricky, but you don’t have to! We make sure your device development efforts comply with relevant standards, giving you peace of mind while you concentrate on your drug’s success.
Comprehensive Device Testing
From determining sample sizes to establishing clinical trial expiration dating, our end-to-end testing services ensure that your device is thoroughly evaluated and stays on schedule for your submissions.
QMS Development
Building a compliant QMS takes time, but we’re here to make it easier. Our team will help you create device-specific SOPs and ensure you have the right systems in place at the right time for lasting success.
FDA Submissions Support
Preparing for FDA Type C meetings and IND submissions can be overwhelming. Let us lead the device regulatory activities and help you prepare for productive interactions with agency.





The EdgeOne Advantage
While many consultants help you build a strategy, we go beyond that—we execute the strategy leveraging our unique infrastructure: a ISO 13485 certified QMS and FDA & EMA registered labs.
With over 100 years of combined leadership experience in compliant device and combination product development, EdgeOne Medical has the expertise and infrastructure to take on the heavy lifting for your team in this critical stage.
We also guide you through the process, so you understand what you need to know, when you need it. This won’t be your last development program, and while you won’t be a device expert at the end of it, you’ll have a fundamental understanding and appreciation for the device development process.
Your Questions, Answered
We understand that navigating Phase II/III can bring up a lot of questions. Here are some common concerns we hear from clients, and how we can help.
-
Our favorite question! The FDA does not define samples size for testing, rather they expect you to determine the sample size required for each test based on risk assessment activities conducted as part of your device development activities. The sample sizes needed for your testing should also be documented within your Verification and Validation plan. Want to learn more? Reach out to see how we can assist you.
-
Yes, your QMS needs to have appropriate SOPs in place to support device development activities OR you need to engage with a provider that offers a QMS compliant with the relevant elements of 21 CFR 820. EdgeOne Medical offers our clients the option to develop their device documentation in their QMS or within our ISO 13485 certified QMS if they don’t have the necessary systems in place, thus preventing delays to their critical programs. Ready to get started? Contact us today!
-
Yes, you absolutely leverage the information developed to support phase II trials for phase III trials. However, you must revisit the completed activities to determine what needs to be updated or repeated, and what additional activities need to be completed. At EdgeOne Medical, we utilized a phase-appropriate approach to device development activities. This means you conduct the device activities necessary to support each clinical phase based on regulatory agency expectations. Have more questions? Feel free to reach out to us!